Division plane specification
Cytokinesis is the physical division of one cell into two that occurs at the end of the cell cycle. To ensure that each daughter cell receives a single copy of the genome, cytokinesis must be tightly coordinated with mitotic chromosome segregation. This coordination is ensured at least in part by signals sent via spindle microtubules. Using the power of genetics and live–cell imaging, we are studying how these spindle-bound proteins and other factors promote efficient division plane specification. We are particularly interested in how division plane specification is differentially regulated in cell types that divide symmetrically versus in cell types that undergo asymmetric cell division, such as occurs during cell fate specification in embryogenesis and in stem cells.
Regulation of contractile ring constriction and robust cell division
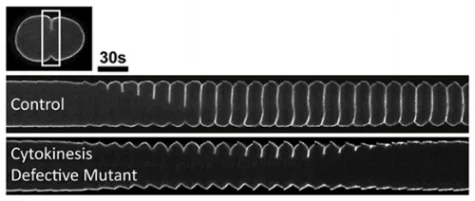
In most animal cells, cytokinesis is accomplished via constriction of an equatorially localized contractile ring composed of filamentous actin and the motor protein myosin II. One focus of our lab is to understand the interplay between stimulatory and inhibitory signaling that promotes efficient constriction of the contractile ring to accomplish cell division. We are also interested in understanding the molecular regulation of cytokinetic diversity among different cell types within a multicellular organism, and the contributions of cell intrinsic and extrinsic signaling.
Imaging-based technology development
To better understand the spatiotemporal regulation of cytokinesis, and enable previously impossible experiments, the lab devotes significant efforts to novel microscopy-based technology development. We have developed a fluidic device to rapidly (<20 seconds) inactivate temperature sensitive mutant proteins at precise times and for precise windows during cell division to determine the first functional molecular timeline for cytokinesis. We are also currently developing a novel optigenetic microscope system to spatially control protein function and determine the important protein sub-populations required for accurate cell division.
The Mighty Worm: a test tube to study cell division
The tiny (~1 millimeter long) soil dwelling round worm Caenorhabditis elegans is a powerful genetic model system to study cell division. The embryos are optically clear and embryonic cell divisions are temporally invariant, and are thus highly amenable to quantitative live-imaging analysis upon gene disruption. These worms are also inexpensive to grow, have a short (3-5 day) generation time, and can be frozen down for long term storage making them fast and easy to work with in the lab. Importantly, the core cell division machinery active in C. elegans is evolutionarily conserved with that in human cells. Thus, our research in C. elegans will help identify novel drug targets to treat human disease.
Genetics of cytokinesis
Forward genetics is a powerful tool for both gene discovery and for identifying complex molecular interactions. In our lab, we work with temperature-sensitive cell division-defective mutations that rapidly (<20 seconds) affect gene function in a conditional manner by simply changing the temperature. At permissive temperature the embryos develop into adulthood, but immediately upon shifting to restrictive temperature cell division fails. The "tunable" nature of these mutants provides a sensitized background to screen for genetic disruptions that either enhance or suppress the cell division defects. Further, by combining this genetic analysis with live-cell imaging, we can quantify the kinetic effects on cytokinesis in vivo.